Honors Human Physiology
BIOSC 1070, NROSCI 1070, MSNBIO 2070
Fall Semester 2020
BIOSC 1070, NROSCI 1070, MSNBIO 2070
Fall Semester 2020
As noted in the synaptic transmission module, glutamate is an excitatory amino acid neurotransmitter. It is one for the most common signaling molecules used in synaptic transmission. This module discuses the types of receptors that the neurotransmitter glutamate binds to, and the effects that occur when such binding takes place.
Glutamate is released by normal synaptic mechanisms described in the synaptic transmission module, and removed from the synaptic cleft by glutamate transporters on the presynaptic nerve terminal.
There are several subtypes of glutamate receptors. Receptors that bind a particular neurotransmitter such as glutamate are not all the same. In fact, binding of a neurotransmitter to one type of glutamate receptor can have vastly different effects than at another.
Typically, receptors with differing responses to the binding of a particular neurotransmitter also have different configurations, and affinities for that neurotransmitter. It thus may be possible for a particular drug to bind to one neurotransmitter receptor “subtype” and not another. This is how neurotransmitter subtypes are differentiated. Often a receptor subtype is named for a particular drug that binds selectively to it, but not other receptor subtypes for the same neurotransmitter.
Most glutamate receptors are ligand-gated ion channels, which are also called ionotropic receptors. However, a few are metabotropic receptors, meaning that binding of glutamate to the receptor activates a second messenger system.
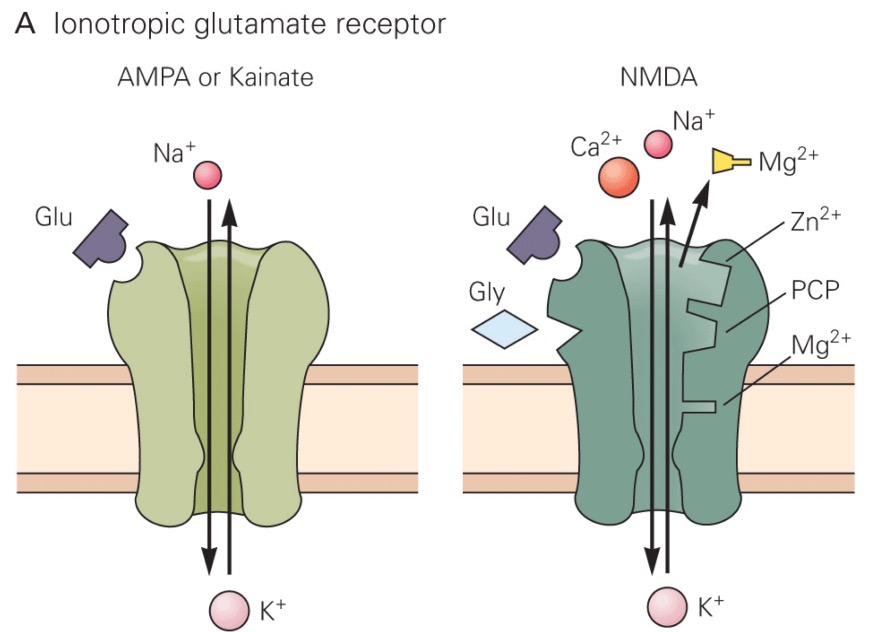 |
There are three subtypes of ionotropic glutamate receptors:
The NMDA receptor is selectively blocked by the drug APV (2-amino-5-phosphonovaleric acid), whereas the AMPA and kainate receptors are both blocked by the drug CNQX (6-cyano-7-nitroquinoxaline-2,3-dione). Consequently, the AMPA and kainate receptor subtypes are sometimes collectively referred to as "non-NMDA" receptors. |
When glutamate binds to either an AMPA or kainate receptor, a cation channel opens that allows both K+ to leave and Na+ to enter the cell. However, the driving force for Na+ to enter the cell predominates, as Na+ is attracted by the negative charge inside the cell, and is also moving down its concentration gradient. As a result, an EPSP occurs inside the neuron.
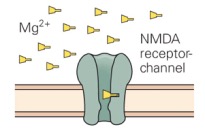 |
The NMDA receptor is unique among ligand-gated channels because its opening depends on both the presence of the agonist (activating ligand) and membrane voltage. At typical resting membrane potentials, the channel of the NMDA receptor is blocked by Mg2+ ions. However, when the membrane is depolarized (such as after glutamate binds to a nearby AMPA receptor), then binding of glutamate to the NMDA receptor can open it. This is because the positive membrane charge forces Mg2+ out of the channel through electrostatic repulsion. |
When the NMDA receptor channel is opened (by the combined binding of glutamate to the receptor and depolarization of the membrane), both Na+ and Ca2+ enter the neuron. The entering Ca2+ can activate various calcium-dependent signaling cascades, including calcium-calmodulin-dependent protein kinases.
The NMDA receptor is also unusual in that it has a binding site for a "co-agonist," glycine. Both glycine and glutamate (and membrane depolarization) must be present for the NMDA receptor channel to open. However, glutamate is the actively controlled agonist for the receptor; glycine may simply be present in activating quantities in the synaptic cleft.
What about kainate receptors? Kainate receptors act much the same as AMPA receptors, but are less common in the brain. No specific function has been assigned to this subtype of glutamate receptors, although they certainly contribute to excitatory neurotransmission in the brain by generating EPSPs in postsynaptic neurons.
Since this is complicated, let's review the material by watching a movie.
If the movie does not play in this window, or you would like to see it in a window of alternate size, download it from this link.
Long-term potentiation is persistent strengthening of synapses based on recent patterns of activity, and is believed to be an important component of learning and memory. NMDA receptors play a key role in generating long-term potentiation in many regions of the nervous system. As noted above, Ca2+ entering through an NMDA receptor can trigger an enzymatic cascade that phosphorylates AMPA receptors (increasing the probability they will open) or causes more AMPA receptors to be translocated to the neuronal membrane. As a result, subsequent releases of glutamate from the presynaptic neuron will cause larger EPSPs in the postsynaptic neuron, increasing the probability that it will generate an action potential. Often AMPA and NMDA receptors are located on the same dendritic spine, and synaptic strengthening through long-term potentiation can be localized to a particular spine or a collection of adjacently-positioned spines.
An opposite phenomenon is long-term depression, which is also often mediated via NMDA receptors. During long-term depression, AMPA receptors are dephosphorylated and fewer AMPA receptors are translocated to the neuronal surface. One function of long-term depression may be to clear old memories. Whether or not activation of an NMDA receptor induces long-term depression or potentiation may rest on the frequency of synpatic inputs, and consequently how much Ca2+ enters through the NMDA receptor channel. If synaptic inputs are frequent and intracellular Ca2+ is high, then long-term potentiation occurs. If synaptic inputs are infrequent and intracellular Ca2+ is low, long-term depression will occur.
Let's review this important concept by watching a movie.
If the movie does not play in this window, or you would like to see it in a window of alternate size, download it from this link.
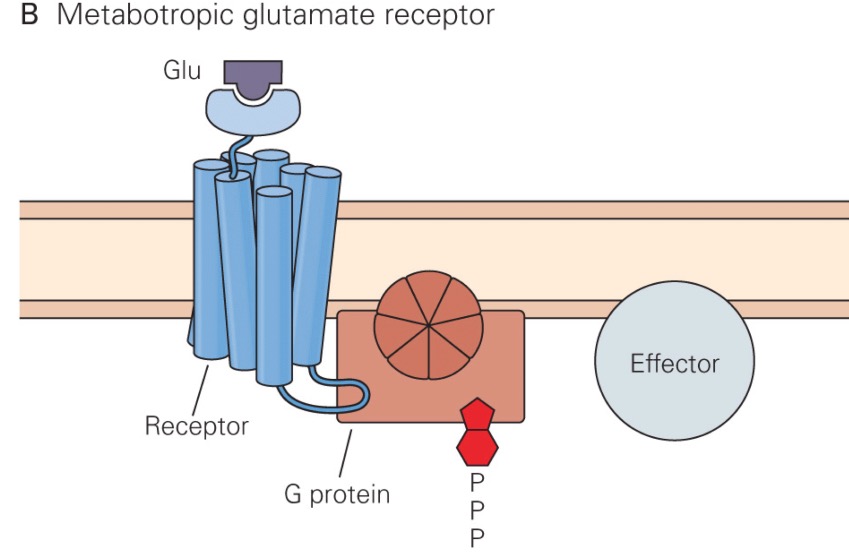 |
In addition to the ionotropic glutamate receptors described above, there are three groups of metabotropic glutamate receptor subtypes. Group 1 metabotropic glutamate receptors are postsynaptic, and apparently modulate the opening of the NMDA receptor. These receptors are usually located on the side of the dendritic spine, away from where the glutamate is released. Thus, they are only activated following a huge presynaptic glutamate release, such that glutamate can diffuse farther than it normally does. When the Group 1 receptors are activated, NMDA receptors allow more Ca2+ to enter the neuron. |
Group II and III metabotropic glutamate receptors are autoreceptors located presynaptically, and modulate how much glutamate is released by the presynaptic neuron during the next action potential. In general, activation of these receptors causes less glutamate to be released subsequently.
Warning: glutamate can be harmful to a neuron's health!
Release of too much glutamate (or administration of too much glutamate agonist) can cause excitotoxicity. Neurons can literally be "excited to death" following exposure to too much glutamate. Although the mechanisms of excitotoxicity are still debated, a predominant theory is that excitotoxicity relates to Ca2+ entry through NMDA receptor channels. When a neuron is continually exposed to glutamate, the cell membrane will be highly depolarized, and via the mechanisms described above an abnormal amount of Ca2+ will enter the cell through NMDA receptor channels. Over time, the effects on enzymatic processes induced by the excessive Ca2+ entry will cause neuronal death.
Excitotoxicity often happens after strokes and other events that cause neurons to die and rupture, releasing their intracellular contents. This causes excessive extracellular glutamate around the nearby neurons that survived the initial traumatic event, such that these nearby neurons eventually die because of excitotoxicity. This process can occur in a wave, with the death of a group of neurons causing subsequent excitotoxicity of their neighbors.
Ischemia of a brain can region also also result in excitotoxicity, as there can be too little ATP synthesis to provide energy for glutamate reuptake into presynaptic terminals via the glutamate transporters.
Since glutamate is the predominant excitatory neurotransmitter in the nervous system, mutations that affect glutamate receptors are associated with a number of serious neurological diseases, including epilepsy. A triggering event for many seizures is too much activation of glutamate receptors. However, since glutamate receptors are so ubiquitous in the nervous system, the efficacy of glutamate receptor antagonists for treating epilepsy is limited, since they cause many side effects. However, a few glutamate receptor antagonists have been approved for epileptic patients, including Perampanel.
A number of drugs of abuse, including Phencyclidine (PCP), also known as "angel dust,", ketamine, and methadone are NMDA receptor antagonists. For example, PCP binds to a site in the NMDA receptor channel pore, thereby partially occluding the movement of ions. These drugs also have other pharmacological actions, but their effects highlight the importance of NMDA receptors in cognitive function.