Honors Human Physiology
BIOSC 1070, NROSCI 1070, MSNBIO 2070
Fall Semester 2020
BIOSC 1070, NROSCI 1070, MSNBIO 2070
Fall Semester 2020
As an introduction, watch this movie from the KhanAcademy.
If the movie does not play in this window, or you would like to see it in a window of alternate size, download it from this link.
The subunits of voltage-gated ion channels change conformation in accordance with membrane potential. This can result in the opening of a pore that allows a specific ion to pass through the membrane.
Two types of voltage-gated channels play a role in producing action potentials: those that allow sodium to cross the membrane (voltage-gated sodium channels) and those that allow potassium to cross the membrane (voltage-gated potassium channels).
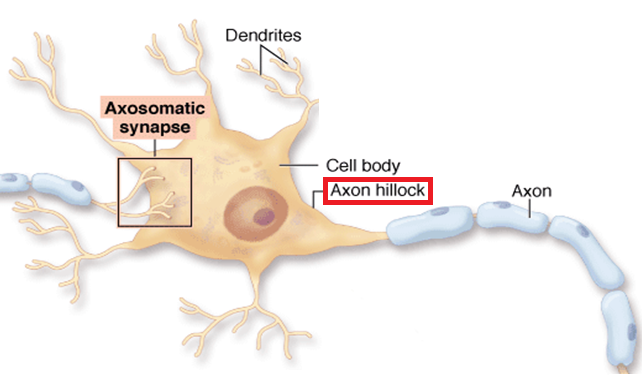 |
Voltage-gated channels are not usually present in the dendrites or the soma, but are concentrated in the initial segment of the axon (axon hillock). Both inhibitory and excitatory postsynaptic potentials are summed in the axon hillock. If the inside of the axon hillock is sufficiently depolarized (becomes less negatively charged), the Na+ channels open and allow Na+ to enter the neuron. Since Na+ concentration is low inside the cell (due to the actions of the sodium-potassium pump) and the inside of the cell is negatively charged, Na+ rushes from the outside to the inside of the cell. |
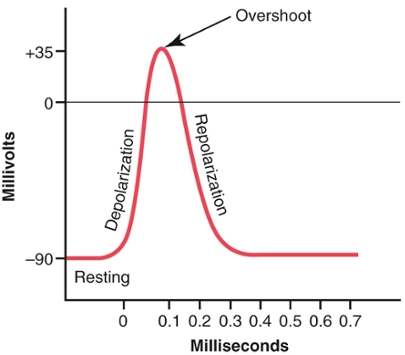 |
When the Na+ channels are open at the axon hillock, the local membrane potential quickly becomes positive. It approaches the equilibrium potential for Na+, but does not reach it before the channels inactivate. When the membrane at the axon hillock becomes depolarized, an opening of voltage-gated K+ channels also occurs. Since K+ is in high concentration inside the neuron, K+ diffuses outward through the channel. However, because of a delay in opening the K+ channels, they open at about the same time that the Na+ channels are closing because of inactivation. Thus, the decrease in sodium entry to the cell and the simultaneous increase in potassium exit from the cell combine to speed the repolarization process, leading to recovery of the resting membrane potential. |
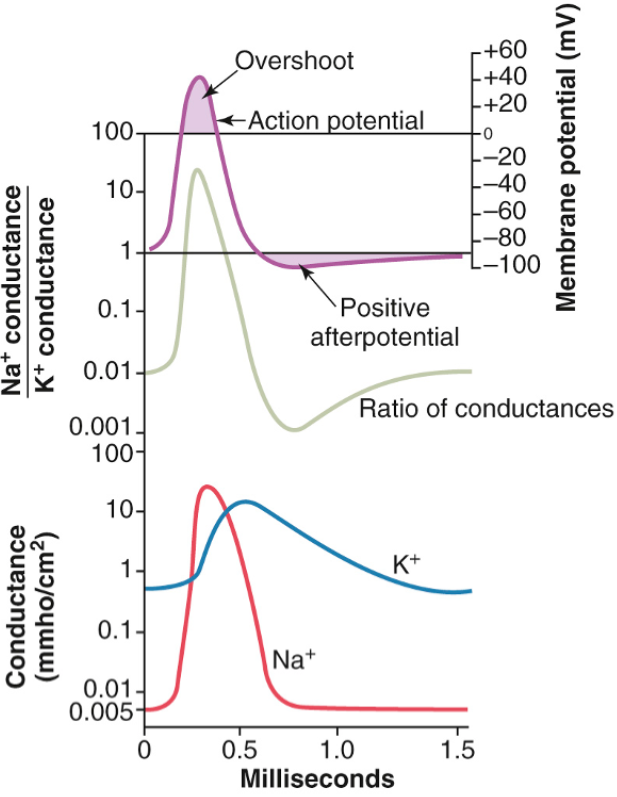 |
This figure summarizes the events that occur during and after the action potential. The bottom of the figure shows the changes in membrane conductance for Na+ and K+ ions. During the resting state, the conductance for K+ ions is 50–100 times greater than the conductance for Na+ ions. This is due to leakage of K+ ions through the leak channels. At the onset of the action potential, Na+ sodium channels open and allow up to a 5000-fold increase in Na+ conductance. The inactivation process then closes the Na+ channels. The onset of the action potential also triggers voltage gating of the K+ channels, causing them to open at the time the Na+ channels close. This produces a 30-fold increase in K+ conductance. At the end of the action potential, the return of the membrane potential to the negative state causes the K+ channels to close slowly. A common feature of action potentials is an afterhyperpolarization. As noted in the membrane potential module, the main factor that sets the resting membrane potential is the movement of K+ ions through leak channels. When the voltage-gated K+ channels are open, the conductance for K+ is higher than during the resting state. As a result, the membrane potential approaches the equilibrium potential for K+ (is more negative than in the resting state), resulting in the afterhyperpolarization. As soon as the voltage-gated K+ channels close, the conductance for K+ is reduced and the membrane potential returns to normal resting values. |
Note that after the resting membrane potential is restored, a short period elapses before the inactivation gates of the voltage-gated Na+ channels open. While the inactivation gate is closed, it is impossible for a new action potential to be elicited. This period is called the absolute refractory period.
As noted above, the voltage-gated K+ channels close slowly after the membrane has been repolarized. Consequently, the K+ conductance is higher (and the neuronal membrane is more hyperpolarized) at the end of the action than in the normal resting state. As a result, it is more difficult to generate the amount of depolarization needed to open the activation gates. This period of higher K+ conductance at the end of an action potential results in a relative refractory period, during which it is possible to elicit an action potential, although a strong excitation is need to do so.
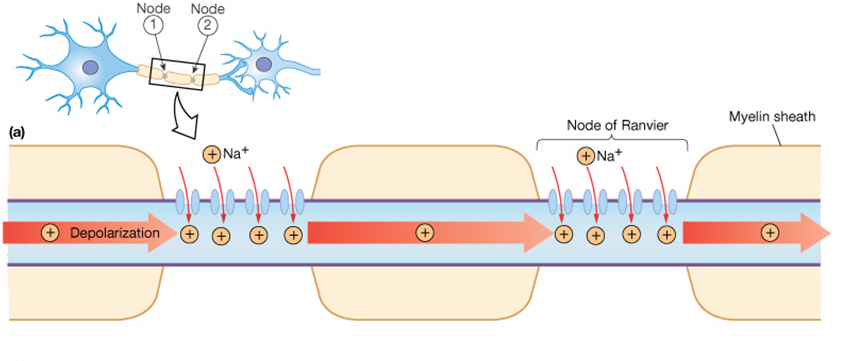 |
Many neurons have myelin surrounding the axon. Myelin is a fatty white substance deposited by glial cells that insulates the axon, decreasing the leak of current through the axonal membrane. The voltage-gated channels described above are located between adjacent myelin sheaths. An unmyelinated area of membrane at the gaps between myelin sheaths, which contains voltage-gated channels, is called a node of Ranvier. |
Myelination allows a bolus of sodium that enters through voltage-gated Na+ channels to move quickly down the axon without leaking out very much. Another action potential occurs at the next node of Ranvier down the axon, refreshing the process. As such, the action potential appears to "leap" between the nodes of Ranvier, in a process called saltatory conduction.
Saltatory conduction allows electrical nerve signals to be propagated long distances at high rates without any degradation of the signal. In addition, the process is energy-efficient, as perturbations in the normal compartmentalization of Na+ and K+ only occur at the nodes of Ranvier. Bear in mind that after an action potential, the sodium-potassium pump has to restore the normal ionic balance across the membrane. Minimizing the need to do this reduces ATP expenditure.
Now you should be able to understand that the refractory period for axons described in the section above has a very practical physiological purpose: it assures that action potentials move in one direction down the axon. When an action potential is generated at one node of Ranvier, the previous node is still in a refractory period. Although sodium ions entering at a node diffuse in both directions down the axon, the previously-activated node cannot generate an action potential. This is key in assuring that an excitatory input to a neuron does not result in a reverberating series of action potentials.
In contrast to myelinated axons, unmyelinated neurons must "refresh" the action potential in every successive patch of membrane. Thus, a repeated entry of Na+ ions and efflux of K+ ions occurs down the axon. The ionic redistribution is restored to the resting state by the sodium-potassium pump, but this requires a large amount of energy.
This may be the reason why unmyelinated axons have a small diameter. If an unmyelinated axon was of large diameter, the surface area would be large and many voltage-gated channels would be needed on the surface. When an action potential occurred, the movement of ions would be large, and a tremendous amount of ATP would be needed to fuel the activity of the sodium-potassium pump to restore ionic balance.
Two major factors govern how quickly an action potential moves downs an axon:
In general, the largest axons are also the most heavily myelinated, and propagate action potentials very rapidly. The smallest axons are unmyelinated and propagate action potentials slowly.
How do the amount of myelination and the diameter of an axon determine its conduction velocity?
Let's turn to the KhanAcademy for an explanation.
Although the action potentials of most neurons resemble those described above, some neurons have action potentials with different properties.
As an example, cerebellar Purkinje cells produce complex spikes, which are very broad and complicated action potentials.
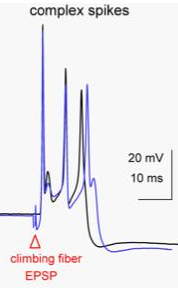 |
As shown to the left, the complex spike has a duration of almost 10 msec. A complex spike is characterized by an initial prolonged large-amplitude spike, followed by a high-frequency burst of smaller-amplitude action potentials. The duration of complex spikes is due to the presence of P-type voltage-gated calcium channels, whose opening contributes to the generation of the action potential. In general, voltage-gated calcium channels open and close slowly, resulting in a prolonged movement of ions. This generates a long-duration action potential. |